alere lyme test kit elisa|lyme disease second elisa test : solutions These enzyme-linked immunosorbent assay (ELISA) test kits are capable of being run in a manual, semi-automated, or fully automated capacity with customized instrumentation packages available to suit individual laboratory needsEnzyme-linked immunosorbent assays identify and calculate substances such as proteins, peptides, antibodies, and hormones. The provided . But what is a composite autoclave? And, how does it function? In this article, key aspects relating to the composite autoclaves, their advantages and disadvantages, together with a broad .
{plog:ftitle_list}
The optimal temperature for sterilization typically ranges between 121°C (250°F) and 134°C (273°F), depending on the type of autoclave and the materials being sterilized.
testing for lyme disease
The ELISA (Enzyme-Linked Immunosorbent Assay) test for Lyme disease is a blood test designed to detect antibodies against Borrelia burgdorferi, the bacterium that .
These enzyme-linked immunosorbent assay (ELISA) test kits are capable of being run in a manual, semi-automated, or fully automated capacity with customized instrumentation packages available to suit individual laboratory needsEnzyme-linked immunosorbent assays identify and calculate substances such as proteins, peptides, antibodies, and hormones. The provided .
All Things Lyme. Gold Standard Diagnostics is the only company offering kits in the U.S. to fulfill Lyme testing requirements for both the Standard and Modified two-tiered testing algorithms. Our four EIA kits and two lmmunoblot kits allow flexibility for your lab to meet your clients' needs.Labcorp test details for Lyme Antibodies, Modified 2-Tier Testing Profile, Serum/Plasma 164226: Lyme Antibodies, Modified 2-Tier Testing Profile, Serum/Plasma | Labcorp Skip to main content
The C6 Lyme ELISA from Immunetics sets a new standard in Lyme test accuracy, and delivers more reliable results sooner and more economically than any other ELISA. It demonstrates 99 percent specificity in an average population – equivalent to the specificity of Western Blot testing and a 10-fold improvement over Whole Cell Sonicate ELISAs.The Alere NMP22 ® Test Kit is an enzyme immunoassay in a 96-well microplate format. The antibodies contained in this assay recognize the head domain of NuMA, nuclear mitotic apparatus protein. NuMA has been shown to be present in malignant tissues at levels more than ten times higher than in normal tissue. 3 The assay is designed to quantify NMP22 in stabilized voided .The first-tier Lyme disease screening enzyme-linked immunosorbent assay (ELISA) used is the Zeus ELISA Borrelia VlsE1/pepC10 IgG/IgM test system. The Zeus ELISA Borrelia VlsE1/pepC10 IgG/IgM test system is designed to detect IgG- and IgM-class antibodies (not differentiated by the assay in the final result) in human sera to VlsE1 and pepC10 .The first test: ELISA (enzyme-linked immunosorbent assay) This blood test is for antibodies against the Lyme disease bacteria. Because it can take some time for your body to produce antibodies, this test isn’t always accurate soon after a person is infected. The second test: Western Blot or a second ELISA. There are two options for the second .
lyme disease testing results
In the study by Wormser et al. , the following assays were used: the C6 Lyme ELISA kit (Immunetics, Inc., Boston, MA) plus either of two WCS ELISAs, i.e., the Wampole IgG/IgM ELISA kit (Alere, Inc., Waltham, MA) or the Vidas II Lyme IgG/IgM screening kit (bioMérieux SA, Marcy l'Etoile, France); the immunoblot assays used were the Lyme IgG and .Test Kit Zeus Elisa" ELISA Lyme Disease Test Serum Sample 96 Tests Abbott Rapid Dx North America LLC 3Z9651 Abbott Rapid Dx North America LLC 3Z9651 - McKesson Medical-Surgical McKessonFind out why IGeneX is the global leader in the research and development of tests that accurately detect Lyme disease, Relapsing Fever, and other tick-borne diseases.At IGeneX, we make it our singular mission to offer best-in-class testing for tick-borne diseases that delivers the most comprehensive and accurate results possible, so you can find the right treatment path to .
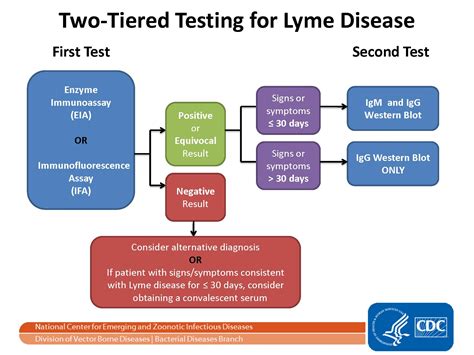
The CDC recommends that doctors first order an ELISA to screen for Lyme disease and then confirm Lyme disease with a Western blot. During the first four-to-six weeks of Lyme infection, these Lyme disease tests are unreliable because most people have not yet developed the antibody response that the test measures.Current serologic testing for Lyme disease follows a 2-tiered algorithm, an enzyme-linked immunosorbent assay (ELISA) followed by supplemental Western immunoblots if the first-tier ELISA is equivocal or positive. 1 We investigated whether a quantitative Lyme ELISA results by itself could inform clinical decision making, without the need to wait for additional immunoblot . The Alere NMP22 ® Test Kit is an enzyme immunoassay in a 96-well microplate format. The antibodies contained in this assay recognize the head domain of NuMA, nuclear mitotic apparatus protein. The antibodies contained in this assay recognize the head domain of NuMA, nuclear mitotic apparatus protein.
The Alere NMP22® Test Kit is an enzyme immunoassay (EIA) for the in vitro quantitative determination of the nuclear mitotic apparatus protein (NuMA) in stabilized voided urine. NuMA is an abundant component of the nuclear matrix proteins . BENEFITS Nuclear Matrix Protein 22 is a common protein .
Evaluation of the most common tick-borne diseases found in the United States, including Lyme disease, human monocytic and granulocytic ehrlichiosis, and babesiosis using the modified 2-tier testing algorithm approach Evaluation of patients with a history of, or suspected, tick exposure who are presenting with fever, myalgia, headache, nausea, and other nonspecific symptoms . Lyme disease is a tick-borne spirochetal infection that affects hundreds of thousands of persons each year in the United States alone [], with a peak in incidence in the school-age and adolescent age groups [].Current serologic diagnosis of Lyme disease consists of a standardized 2-tiered testing protocol: a whole-cell sonicate (WCS) enzyme immunoassay .B. burgdorferi IgM (Lyme) ELISA Test kit Lyme disease ELISA Test kit Intended Use Borrelia burgdorferi IgM ELISA kit is an ELISA for the qualitative detection of IgM antibodies to Borrelia burgdorferi in human serum. This ELISA test kit should be used for patients with signs and symptoms that are consistent with Lyme disease and can be used to .
Lyme disease; this requires a highly specific test. Thus, Lyme disease is an illness where sequential testing (beginning with a sensitive initial test followed by a specific test), such as the CDC's two-tier protocol, is appropriate. Unfortunately, in clinical settings, the true sensitivity of the 2-tier test system using standard commercial . The Alere NMP22® Urine Collection Kit containers are intended for the collection, stabilization and transport of human urine to be tested using the Alere NMP22® Test Kit. Product exclusively available for export to select markets.
Lyme Disease IgM ELISA Test Kit which is designed to detect IgM class antibodies to B. burgdorferi in human sera. For more information about these Lyme Disease ELISA Test Kits, Rapid Tests, IFA Kits, CLIA Test Kits, or Serology tests, please see our website home page, or contact our Customer Service Representatives at 818-591-3030 . .Alere NMP22® Test 1 of 15 N Caution: U.S.A. Federal law restricts this device to sale and distribution by or on the order of a physician, or to a clinical laboratory, and use is restricted to, by, or on the order of a physician. INTENDED USE The Alere NMP22® Test is an enzyme immunoassay (EIA) for the in vitro quantitative determination of the nuclear mitotic apparatus .
All CE IVD Elisa Kits; Allergy CE IVD Elisa Kits; Fertility & Steroids CE IVD Elisa Kits; Hepatitis CE IVD Elisa Kits; HIV CE IVD Elisa Kits; . Ehrlichia EHR Antibody, Heartworm CHW Antigen Rapid Test Kit Increase Quantity of Canine Lyme Antibody, Anaplasma ANA Antibody, Ehrlichia EHR Antibody, Heartworm CHW Antigen Rapid Test Kit. Price: .
lyme disease testing guidelines
Newly-cleared test is a game changer for the diagnosis of an often debilitating disease. MILPITAS, Calif., September 4, 2024 – ID-FISH Technology, Inc., a leading provider of diagnostic tools for the detection of tick-borne diseases, today announced that its Lyme ImmunoBlot test has received FDA clearance. The name of the test is iDart™ Lyme IgG ImmunoBlot Kit. The iDart .Age restrictions apply: test taker must be 18 years or older to receive lab results. Use the CVS Health At Home Lyme Disease Test Kit to get accurate and comprehensive results in the privacy and comfort of your own home. Simply collect your blood sample using this convenient kit, mail it to the lab, and receive your results through a secure online portal in just a few days.17 steps for conventional ELISA kits : 1. Washing of coated plates 2. Reconstitution of standard protein 3. Addition of diluent to standard wells: 1. Rehydration of standard and sample wells on plate : 4.Titration of standard curve 5. Addition of sample diluent: 2. Sample addition : . Combination Lyme Disease IgG/IgM ELISA Test Kit, designed to detect IgM and IgG class antibodies to B. burgdorferi in human sera. For more information about these Lyme Disease ELISA Test Kits, Rapid Tests, IFA Kits, CLIA Test Kits, or Serology tests, please see our website home page, or contact our Customer Service Representatives at 818-591 .
Samples negative by this first-tier test do not require further testing. . It is intended for use in testing of samples which have been found positive or equivocal 1 st tier ELISA. Lyme Disease Forms for Printing: Lyme Results Release Form; Lyme Request Form; Lyme Lab Brochure ; CAP Certificate; DOH Certificate 2024-2025 . Specimen Shipping .
lyme disease test interpretation
laboratory autoclave manufacturers in mumbai
Discover the autoclave molding process, its advantages, and applications across industries such as aerospace and automotive. Learn how it enhances composite material properties.
alere lyme test kit elisa|lyme disease second elisa test